Law 4 Headgear Trial

World Rugby Law 4 Headgear Trial
World Rugby has developed a trial process to enable the assessment of headgear devices which, according to the manufacturers have been designed to achieve specific, quantifiable medical purposes.
This trial has been established as an indication of World Rugby’s support of innovative technologies designed to manage and improve player welfare. Whilst World Rugby hopes that such innovative technologies are ultimately beneficial for player welfare, there is no guarantee that this will be the case and World Rugby reserves the right to terminate the trial at any time if it feels that its continuance will put players at an increased risk of injury or otherwise damage player welfare or the game of Rugby.
It is important to note at the outset that World Rugby makes no claim as to the ability of the headgear devices to achieve the claims made by the manufacturers.
Any and all claims are made solely by the manufacturer, World Rugby will carry no liability in relation to the use of the headgear devices and World Rugby’s role in the process is limited to:
- ensuring that nothing is worn which to the knowledge of World Rugby may increase the risk of injury to players and
- enabling an assessment of claims of advances in technology, which may have a benefit for players in line with world Rugby priorities, by permitting use on the field of play with players’ knowledge and consent.
For the avoidance of confusion, ASSESSMENT is used to describe World Rugby’s exploration of whether this process should become a permanent part of Law 4 and by association Regulation 12 whereas TRIAL describes the actual process that a device will go through in meeting the claims made by the manufacturer.
The trial process has been developed on the assumption that player behaviour will not change as a result of using these devices and a project will be undertaken to ensure that this is the case.
The intention of this assessment is to encourage innovation in the development of rugby headgear which advances player welfare while maintaining the spirit of Law 4 and Regulation 12. This spirit is to permit the use of padded clothing without enabling the use of rigid materials which it is thought might alter how the game is played.
Please note that this trial is for headgear only and is not to be used for any other body padding.
The Application Process
There are several stages that must be followed before permission will be considered for inclusion in the assessment. These stages are undertaken by a combination of World Rugby, a World Rugby recognised Test House and an independent body established to assess medical devices. Figure 1 provides an indicative flow chart of the stages that must be achieved to get the device on the field of play.
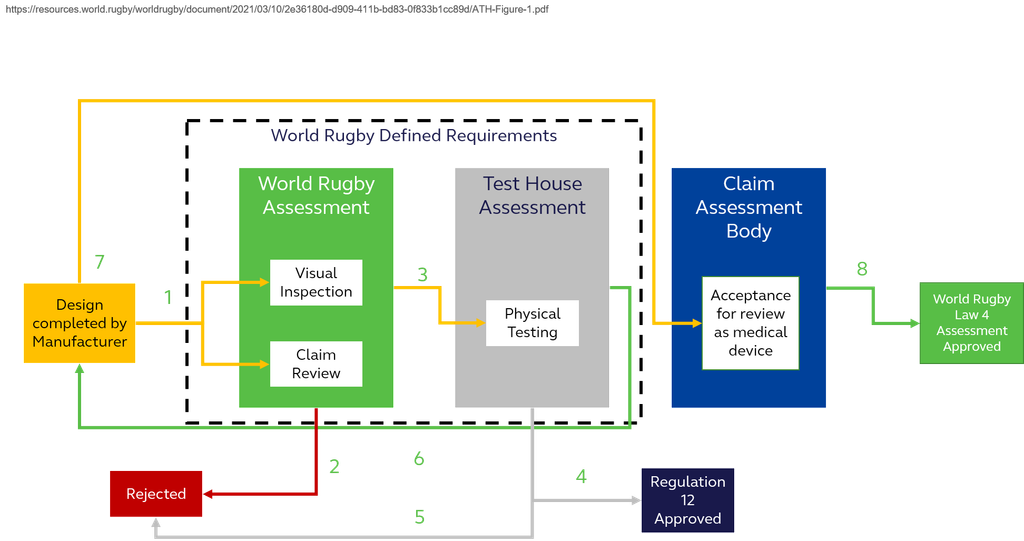
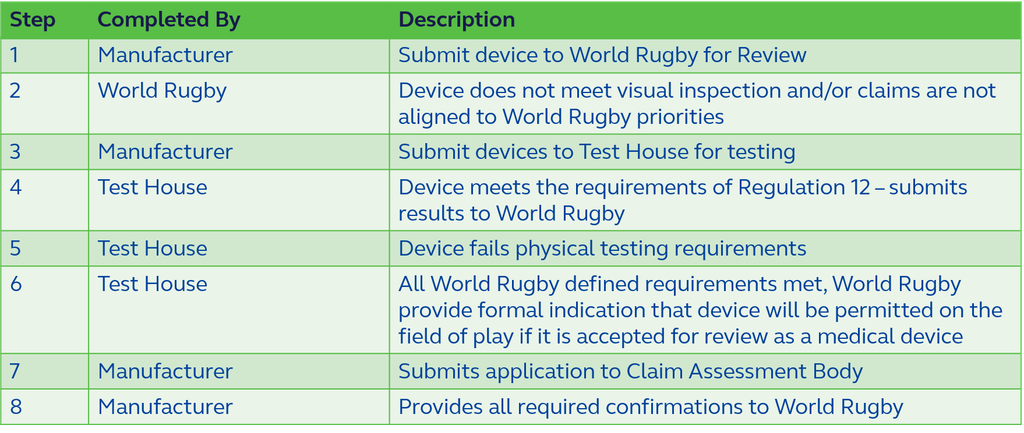
An indication of the processes indicated for recognised claim assessment bodies is available in Section below.
World Rugby Assessments
Visual Assessment
World Rugby will require a physical sample of the device to be sent to them for a visual assessment to ensure that there is nothing included in the design of the device which may be considered to increase the risk of injury to players. As this whole assessment process is new, World Rugby recognise that it is not possible to account for all potential design features and this visual assessment is in place only to prevent any unforeseen aspects being permitted on the field of play.
Assessment of medical claims being made
To ensure that the medical claims being made are aligned with World Rugby’s priorities in the area of player welfare they must be approved in advance of any formal application process starting. Manufacturers are free to include other claims as part of their application but areas identified by World Rugby must be included.
Independent Test House Assessments
The physical testing requirement is designed to use that which is currently in place within Regulation 12 for headgear but to make adjustments that enable the innovation required to provide a medical device that advances player welfare.
All requirements within Regulation 12 must continue to be met except for the following aspects:
Impact Attenuation – The limits for impact attenuation identified in Regulation 12, Performance Specification for Specific Items of Players’ Clothing, Section 3.4.1 do not apply for this process. The device must be tested as per the Regulation but should be done so at the following range of drop heights as an indicative exercise (note where the test device cannot reach the 0.6m and 0.9m drop heights, these can be ignored):
- 0.15m
- 0.3m (the height described in the Regulation)
- 0.45m
- 0.6m
- 0.9m
Surface Stiffness/Elasticity – The test protocol listed in Appendix 3 is to be used and the results must not exceed 250N.
Density – There is no density limitation on devices to be included in the trial.
Sandwich Construction - The prohibition of sandwich construction does not apply for the purposes of this trial. However, devices must be tested for impact attenuation both as intended to be worn and inside out and the results compared. The results when tested inside out shall not exceed 110% of the results when tested as intended to be worn. The tests with the device inside out need only be performed at a drop height of 0.3m.
A full explanation of all the test requirements is available below.
If the device meets all the requirements of Regulation 12, and the associated Performance Specification, then it is not required to go through this trial process and can be worn on the field of play once it complies with the all the requirements of the regulation.
Independent Body
Participation in the trial will only be permitted if the device is part of a claim validation process recognised by World Rugby. This validation process must consider the headgear a medical device (or other similar specific categorisation identified by World Rugby) and have a robust and independent process of examination.
World Rugby recognise that the process to achieve validation requires assurances to any assessment body that an adequate research environment is available to assess the validity of the device.
Where all other requirements are met, World Rugby will provide provisional support to enable the device to be used in matches. This provisional support is provided to enable a complete application to be made to the validation process. Only when the device has been accepted (and World Rugby provided with evidence of this acceptance) into the validation process will its use be permitted on the field in any match.
Post-Trial Acceptance
Figure 2, below, shows the stages to be followed post acceptance into the trial phase.
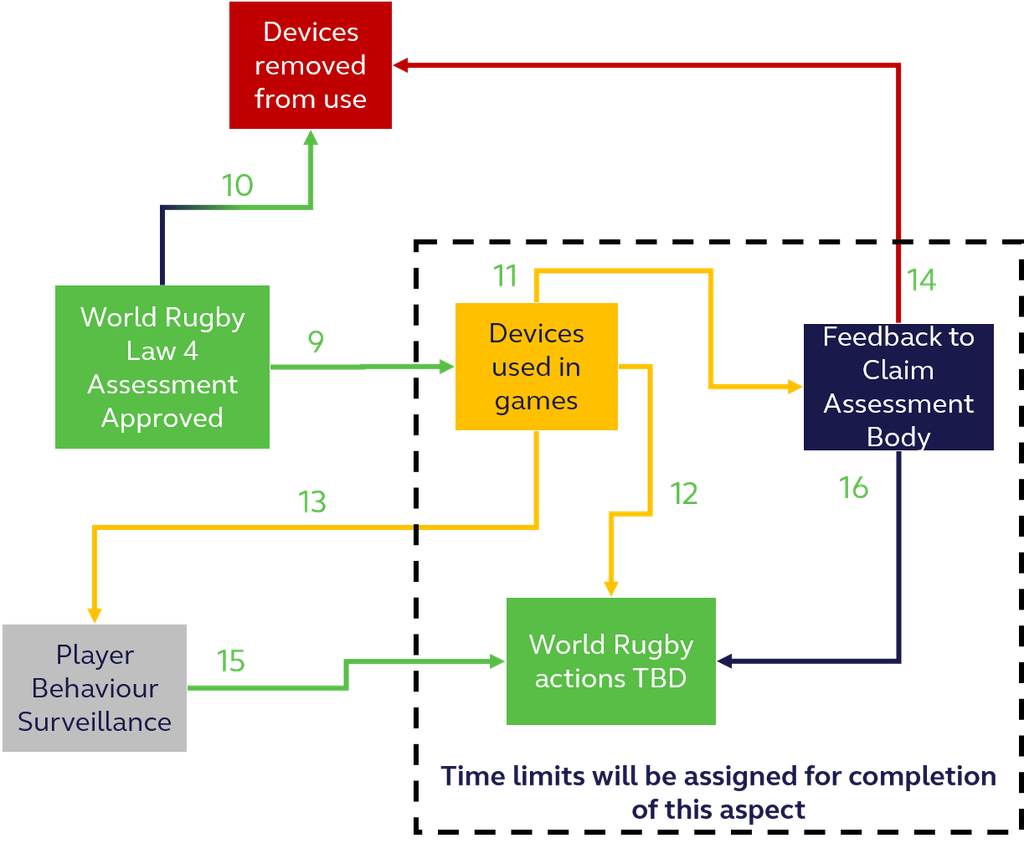
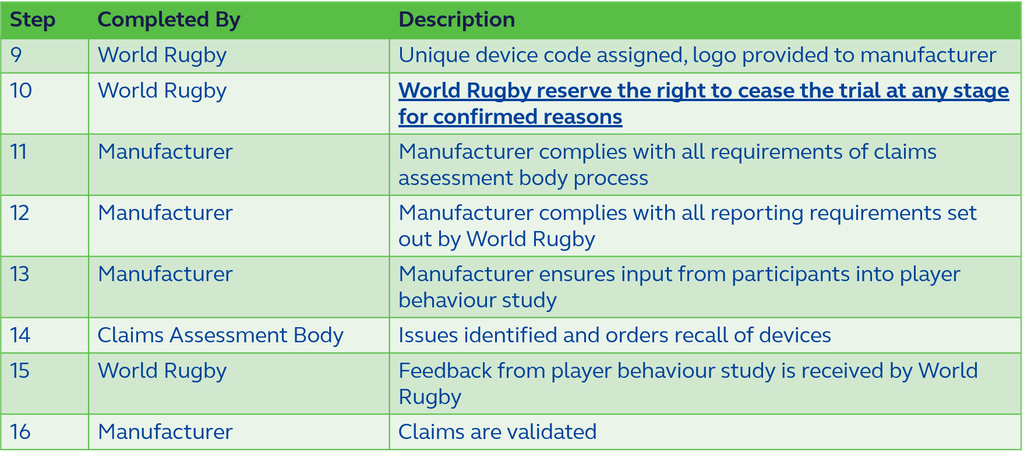
Trial Branding
All headgear devices, which are part of the trial will be provided with a unique identification code. This must be displayed in a legible fashion along with the World Rugby Trial Approved Logo on the external surface (towards the rear of the device is recommended) of the device. The logo must be at least 100mm at its narrowest point and the text displaying the code at least 60mm tall.
If the logo and code are printed on the crown of the device, they must also be printed elsewhere on the device.
A World Rugby Trial Approved label (these must be purchased from World Rugby) must also be inserted on the interior of the device as per Regulation 12 compliant devices.
Behavioural Considerations
As part of the overall process of assessing the viability of these devices for rugby, World Rugby has a duty of care to ensure that their use does not result in behavioural changes in players that increases their or other players’ risk of injury. Applicants should include assessment of these changes in their studies. In additoin, to identify whether this is the case or not, World Rugby will establish a surveillance study that will include player behaviour. The final aspect of the application is to ensure that all participants in the trial used to validate the claims consent to taking part in the World Rugby behavioural study.
Recognised claims assessment bodies
| Stage | World Rugby Description | Food and Drug Adminsitration (FDA) Description |
| 7 | Application to Claims Assessment Body | De Novo Application / 510K |
| 8 | Acceptance for review as medical device | Protocol Approval |
| 9 | Claims made validated | Certified |
Variation from Regulation 12
The requirements of Regulation 12 as it pertains to headgear must be met in full, except for all the following aspects:
- Impact attenuation – the limit identified in Regulation 12, Performance Specification for Specific Items of Players’ Clothing, Section 3.4.1 is not required. However, the test must be completed, at the indicated drop heights, both as the device is intended to be worn and inverted (using the same process, described in 4.3 of the Regulation, for both) and the results shall not differ from each other by more than ±10%.
- Density – the limit identified in Regulation 12, Schedule 1, Section 1(d) is not applicable.
- Sandwich Construction – the prohibition of sandwich construction for materials not applicable
- Stiffness – the stiffness of the device as constructed for use must be ≥XN/≥X% when assessed using the test method described below.
Test Procedure for determining the stiffness of the combination of materials used in headgear
The purpose of this test is to determine the characteristics of the composite parts of the device as they behave in providing the impact attenuation properties of the device.
Preparation
The device must be separated at the seams so that the individual parts can be laid flat on a level surface. If this is not possible, then incisions must be made to allow this as much as possible while not cutting through a performance aspect of the device and providing portions that are large enough to perform the testing as required.
Testing must take place at the ambient temperature and humidity indicated in Regulation 12 Schedule 1.
Procedure
The test procedure detailed in ISO 2439 Method C must be used. The indenter must have a diameter of 50mm (±1mm) and comply with all other requirements of the test procedure.
The protocol must include at least 5 locations – those indicated for testing of impact attenuation within Regulation 12, Schedule 1 (or as close as possible allowing for the conditions below) of the crown, forehead and temple areas, at least 2 additional locations as chosen by the tester to be representative of the overall performance of the device. One of these must be at least 200mm from any other test location measured along the surface of a fully assembled device. All additional locations (as deemed necessary by the tester) must not overlap with any other test location. For the avoidance of doubt the test location includes any area under the impactor during a test.
Each test locations must:
- Not overlap the edge of the device, any stitching or seams.
- Not overlap a hole in the device that has an area ≥110 mm2.
- Overlap 2 nodules unless one of the nodules has an upper surface area ≥2,000mm2.
- Part of the edge of the impactor must be in contact with the surface of the device at the start of the application of force
Terms and Conditions
Read the Terms and Conditions for this trial.




